The in vitro H295R Steroidogenesis Assay – OECD 456
The steroidogenesis test is a screening test that identifies endocrine disrupting chemicals (EDs, Endocrine disruptors). EDs are natural or man-made chemicals that can mimic, block, or disrupt the action of hormones, which can lead to a variety of health problems. EDs are widespread and found in many everyday products including some cosmetics, food and beverage packaging, toys, carpets, and pesticides. Unfortunately, it is not possible to completely remove EDs, but it is important to identify such substances in order to reduce exposure and the risk of potential health effects. The test conducted in accordance with guideline OECD 456 allows the detection of substances influencing the production of 17β-estradiol (E2) and testosterone (T). This is a qualitative study that determines the potential of a xenobiotic to both inhibit and stimulate the synthesis of E2 and T.
What’s important, the steroidogenesis test is an in vitro test that does not require the use of animals.
Monocyte activation test – ISO/Pharmacopoeia
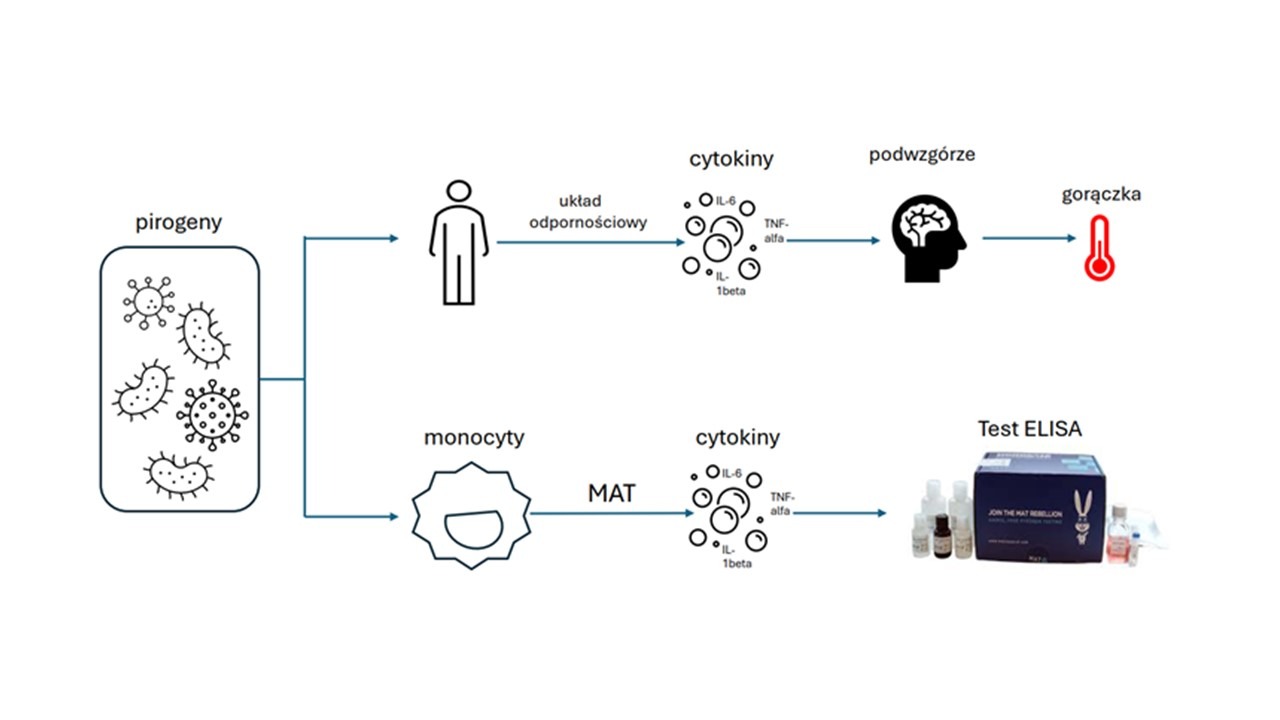
Parenterally administered medicinal products, implantable medical devices used in humans or animals (veterinary products) must not cause life-threatening febrile reactions. Fever-causing factors are pyrogens, which include endotoxins (mainly lipopolysaccharides of the cell wall of Gram-negative bacteria) and non-endotoxin pyrogens (components of Gram-positive bacteria; viral, yeast and fungal pyrogens; rubber particles, microplastics, organic dust, packaging materials). Assessment of the degree of pyrogenic contamination (endotoxins, non-endotoxin pyrogens) of sterile parenteral drugs and medical devices is a universally required quality control measure, is of great importance for patient safety and is regulated by the European Pharmacopoeia (EP).
The principle of the monocyte activation test (MAT) is the detection of proinflammatory cytokines released by human monocytes upon stimulation with pyrogenic substances. The MAT test is an alternative to animal testing, i.e. the rabbit pyrogen test (RPT)