Branch in Pszczyna – Toxicological and Ecotoxicological Research Centre
Toxicology Research Group



Toxicology Research Group consists of the following sections:
- Laboratory of Experimental Toxicology
- Laboratory of Diagnostics and Alternative Methods
The Toxicology Research Group consists of a team of specialists in the field of medical sciences, health sciences, life sciences, veterinary sciences and agricultural sciences, engaged in assessing the toxicity of chemical substances, their mixtures, and biological substances. The professional team and modern equipment allow us to provide research services at the highest level.
We perform toxicological studies noting the priority of alternative studies (in chemico, in vitro or ex vivo) over the studies performed on vertebrate animals.
The studies are performed to assess the safety of chemical and biological substances contained in medicinal products, medical products, plant protection products, biocidal products, food and feed additives, cosmetics, industrial wastes and industrial chemical agents. The purpose of these studies is to determine the harmfulness of the substance in relation to human and animal health. The studies conducted in the Toxicology Research Group enable the development of models that allow tracking the fate of chemical substances in the body, and explaining the mechanisms of their toxic effects.
The studies are conducted under the Good Laboratory Practice quality system according to methods: OECD, EU, SCCS, ISO, EMA, FDA, and their results are used for registration purposes, licensing, as well as other regulations in the field of industrial chemicals.
The list of studies that can be performed at the Toxicology Research Group:
In vitro, ex vivo, in vivo eye corrosion/ irritation studies
Isolated chicken eye test method for identifying
I) chemicals inducing serious eye damage,
II) chemicals not requiring classification for eye irritation or serious eye damage.
Short time exposure in vitro test method for identifying I) chemicals inducing serious eye damage
II) chemicals not requiring classification for eye irritation or serious eye damage.
The test method used within the study on the model of reconstructed human cornea-like epithelium (RhCE), used for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage.
Reconstructed human cornea-like epithelium (RHCE) test method for eye hazard identification.
Delayed hypersensitivity assessment test using the closed-patch method (Buehler Test).
ISO 10993-10
In vitro, ex vivo, in vivo skin corrosion/ irritation studies
In vitro skin corrosion: transcutaneous electrical resistance test (TER).
In vitro skin corrosion: reconstructed human epidermis (RHE) test method.
In vitro skin irritation. The test on the model of reconstructed human epidermis.
Acute skin irritation/ corrosion test.
Biological evaluation of medical devices – Test for skin irritation in vitro.
ISO 10993-23
In vitro, in chemico, in vivo skin sensitisation studies
Skin sensitisation: local lymph node assay (LLNA): BrdU-ELISA.
In chemico skin sensitisation, direct peptide reactivity assay (DPRA).
In vitro skin sensitisation: the test method with use of ARE-Nrf2 Luciferase.
In vitro skin sensitisation: Human cell line activation test (H-CLAT).
Skin sensitisation study.
Delayed hypersensitivity assessment test using the closed-patch method (Buehler Test).
ISO 10993-10
In vitro cytotoxicity studies
In vitro cytotoxicity test.
In vitro phototoxicity
Phototoxicity – In vitro 3T3 NRU phototoxicity test.
In vitro Phototoxicity – Reconstructed Human Epidermis Phototoxicity test method.
In vitro Pyrogen Test
Monocyte Activation Test
ISO/ Farmakopea
In vitro, in vivo studies identifying endocrine-disrupting substances
Stably Transfected Human Estrogen Receptor-α Transactivation Assay for Detection of Estrogenic Agonist and antagonist Activity of Chemicals using the hERα-HeLa-9903 cell line.
Uterotrophic Bioassay in Rodents
A short-term screening test for oestrogenic properties.
Hershberger bioassay in rats. Short-term screening test of (anti-) androgenic properties.
H295R Steroidogenesis Assay
In vitro, in vivo acute toxicity
Use of the 3T3 neutral red uptake cytotoxicity test to estimate starting doses for acute oral systemic toxicity tests.
Acute oral toxicity test.
Acute oral/ intraperitoneal/ subcutaneous/ intravascular toxicity test – acute toxic class method
Acute dermal toxicity test.
Acute inhalation toxicity test.
Acute inhalation toxicity – acute toxic class method.
Maximum tolerated dose test (MTD).
Single dose toxicity study in rodents.
ISO 10993-11
In vivo subacute, subchronic and chronic studies
Dose range finding test (DRF).
Repeated dose 28-day oral/ intraperitoneal/ subcutaneous toxicity study in rodents.
Repeated dose dermal toxicity: 21/ 28 day study.
Repeated dose 90-day oral toxicity study in rodents.
Subchronic dermal toxicity: 90-day study.
Chronic toxicity studies.
Combined chronic toxicity/ carcinogenicity studies.
In vivo neurotoxicity studies
Neurotoxicity study in rodents.
In vivo developmental and reproduction studies
Prenatal developmental toxicity study.
Reproduction/ developmental toxicity screening test
Combined repeated dose toxicity study with the reproduction/ developmental toxicity screening test.
Extended one-generation reproductive toxicity study in rats.
Two-generation reproduction toxicity study.
In vitro, in vivo genotoxicity studies
Bacterial reverse mutation test (AMES test, microplate format).
In vitro micronucleus test on the mammalian cell line.
In vitro mammalian cell gene mutation tests using the HPRT genes.
In vivo mammalian erythrocyte micronucleus test.
In vivo mammalian cell gene mutation test using the thymidine kinase gene.
In Vivo Mammalian Alkaline Comet Assay.
In vivo carcinogenicity studies
Badanie rakotwórczości
Combined chronic toxicity/ carcinogenicity studies.
In vivo neurotoxicity studies
Local tolerance test.
In vivo local tolerance studies
Toxicokinetics/pharmacokinetics study.
OECD Nr 417/ EU B.436/ ICH S3A
Other services
Toxicological studies conducted according to the procedures agreed with the customer
Haematological studies
Biochemical studies
Hormone identification
Tissue and organ collection
Preparing histopathology slides and their scanning
Since 2000, we have been holding Good Laboratory Practice Certificates obliging us to use strictly defined research procedures, and the results obtained in the studies have a high practical and scientific value. We were the first in Poland to receive full AAALAC International accreditation granted by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International Council. This accreditation confirms compliance with the highest standards related to ensuring the welfare of laboratory animals.
List of equipment
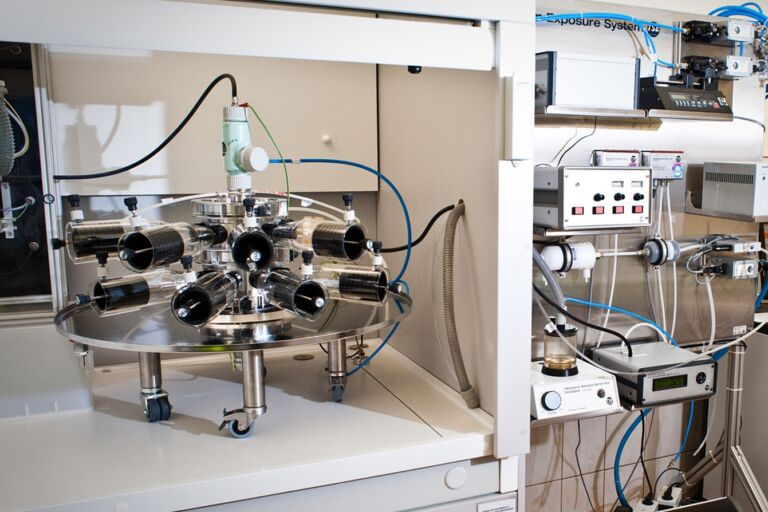



- inhalation exposure system
- metabolic cage system with e-Chillers
- behavioural testing kits: motor activity testing; assessment of animal pain response; apparatus for measuring grip strength of fore and hind limbs
- apparatus for inhalation anaesthesia of animals
- cytometer
- hematological analyser
- biochemical analyser
- histology scanner
- unique Superfusion apparatus used for studies on isolated chicken eye according to OECD no. 438
- tissue processo
- paraffin embedding station
- automatic stainer for histology slides
- equipment for cell culture: BSLII chambers, microscopes, incubators, etc.
Collaboration opportunities #FutureMadeOrganic
WHAT ARE OUR CORE RESEARCH DOMAINS?
Toxicology • Pharmacy • Medical devices • Pharmaceutical products • Supplements • Cosmetics • In vivo studies • Ex-vivo studies • In vitro studies • Safety • Sustainability • Environmental protection • GLP • ISO 10993 • OECD TG • REACH
WHY DO YOU NEED US?
We conduct toxicological studies with a strong focus on alternative methods (in chemico, in vitro, ex vivo) over traditional animal testing. Our studies assess the safety of chemical and biological substances in pharmaceuticals, medical products, plant protection products, biocides, food and feed additives, cosmetics and industrial chemicals. The goal is to evaluate the potential risks to human and animal health and to understand the substances’ behaviour and mechanisms of toxicity.
WHERE ARE WE READY TO COLLABORATE?
Cluster 1 • Cluster 5 • Cluster 6 • Widening instruments • EIC / EIT • R&D Projects
Our Expert

Head of the Toxicology Research Group
WHO IS OUR GO-TO EXPERT?
Graduate of the Faculty of Biology and Environmental Protection at the University of Silesia, she holds a PhD in pharmaceutical sciences, with a specialization in toxicology, awarded by the Scientific Council of the Faculty of Pharmacy at the Medical University in Poznań.
CONTACT
e-mail: inga.mrzyk@ipo.lukasiewicz.gov.pl
Phone: +48 32 210 30 81 ext. 108, +48 781 758 000